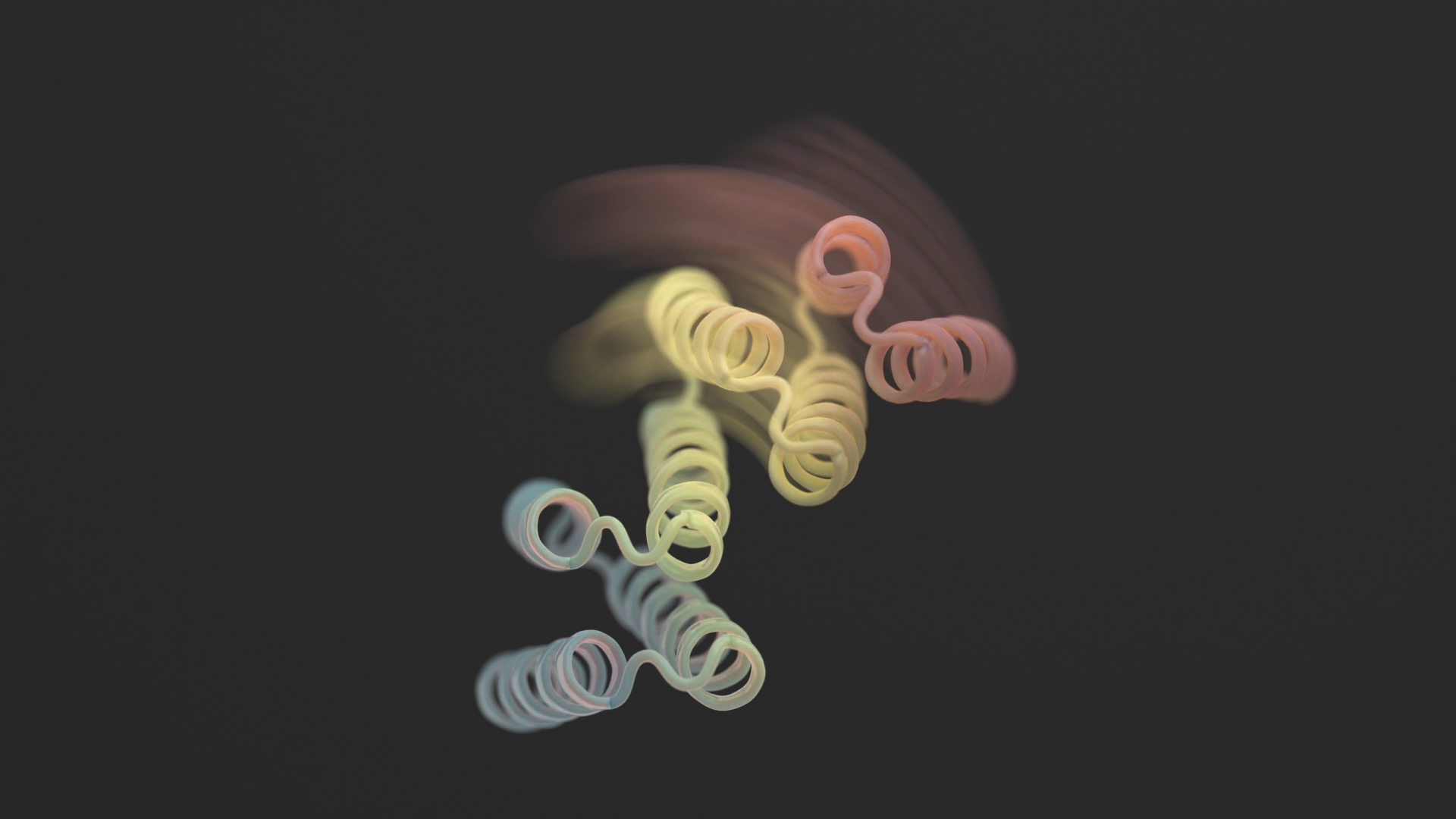We regularly consider proteins as immutable 3D sculptures.
That’s not fairly proper. Many proteins are transformers that twist and alter their shapes relying on organic wants. One configuration could propagate damaging alerts from a stroke or coronary heart assault. One other could block the ensuing molecular cascade and restrict hurt.
In a manner, proteins act like organic transistors—on-off switches on the root of the physique’s molecular “pc” figuring out the way it reacts to exterior and inner forces and suggestions. Scientists have lengthy studied these shape-shifting proteins to decipher how our our bodies perform.
However why depend on nature alone? Can we create organic “transistors,” unknown to the organic universe, from scratch?
Enter AI. A number of deep studying strategies can already precisely predict protein constructions—a breakthrough half a century within the making. Subsequent research utilizing more and more highly effective algorithms have hallucinated protein constructions untethered by the forces of evolution.
But these AI-generated constructions have a downfall: though extremely intricate, most are fully static—primarily, a kind of digital protein sculpture frozen in time.
A brand new examine in Science this month broke the mildew by including flexibility to designer proteins. The brand new constructions aren’t contortionists with out limits. Nevertheless, the designer proteins can stabilize into two totally different varieties—assume a hinge in both an open or closed configuration—relying on an exterior organic “lock.” Every state is analogous to a pc’s “0” or “1,” which subsequently controls the cell’s output.
“Earlier than, we may solely create proteins that had one steady configuration,” mentioned examine creator Dr. Florian Praetorius on the College of Washington. “Now, we will lastly create proteins that transfer, which ought to open up a rare vary of purposes.”
Lead creator Dr. David Baker has concepts: “From forming nanostructures that reply to chemical compounds within the atmosphere to purposes in drug supply, we’re simply beginning to faucet into their potential.”
A Protein Marriage Made in AI
A fast little bit of biology 101.
Proteins construct and run our our bodies. These macromolecules start their journey from DNA. Genetic data is translated into amino acids, the constructing blocks of a protein—image beads on a string. Every string is then folded into intricate 3D shapes, with some elements sticking to others. Known as secondary constructions, some configurations seem like Twizzlers. Others weave into carpet-like sheets. These shapes additional construct on one another, forming extremely refined protein architectures.
By understanding how proteins acquire their shapes, we will doubtlessly engineer new ones from scratch, increasing the organic universe and creating new weapons towards viral infections and different ailments.
Again in 2020, DeepMind’s AlphaFold and David Baker lab’s RoseTTAFold broke the structural biology web by precisely predicting protein constructions primarily based on their amino acid sequences alone.
Since then, the AI fashions have predicted the form of virtually each protein identified—and unknown—to science. These highly effective instruments are already reshaping organic analysis, serving to scientists shortly nail down potential targets to fight antibiotic resistance, examine the “housing” of our DNA, develop new vaccines and even make clear ailments that ravage the mind, like Parkinson’s illness.
Then got here a bombshell: generative AI fashions, similar to DALL-E and ChatGPT, supplied a tantalizing prospect. Fairly than merely predicting protein constructions, why not have AI dream up fully novel protein constructions as an alternative? From a protein that binds hormones to manage calcium ranges to synthetic enzymes that catalyze bioluminescence, preliminary outcomes sparked enthusiasm and the potential for AI-designed proteins appeared limitless.
On the helm of those discoveries is Baker’s lab. Shortly after releasing RoseTTAFold, they additional developed the algorithm to nail down useful websites on a protein—the place it interacts with different proteins, medication, or antibodies—paving the best way for scientists to dream up new drugs they haven’t but imagined.
But one factor was lacking: flexibility. A lot of proteins “code shift” in form to alter their organic message. The consequence may actually be life or dying: a protein known as Bax, for instance, alters its form right into a conformation that triggers cell dying. Amyloid beta, a protein concerned in Alzheimer’s illness, notoriously takes a unique form because it harms mind cells.
An AI that hallucinates related flip-flop proteins may edge us nearer to understanding and recapitulating these organic conundrums—resulting in new medical options.
Hinge, Line, and Sinker
Designing one protein on the atomic stage—and hoping it really works in a dwelling cell—is difficult. Designing one with two configurations is a nightmare.
As a unfastened analogy, consider ice crystals in a cloud that finally type into snowflakes, every one totally different in construction. The AI’s job is to make proteins that may shift between two totally different “snowflakes” utilizing the identical amino acid “ice crystals,” with every state equivalent to an “on” or “off” swap. Moreover, the protein has to play good inside dwelling cells.
The staff started with a number of guidelines. First, every construction ought to look vastly totally different between the 2 states—like a human profile standing or sitting. They may test this by measuring distances between atoms, defined the staff. Second, the change must occur quick. This implies the protein can’t fully unfurl earlier than piecing itself again collectively into one other form, which takes time.
Then there are some groundskeeping pointers for a useful protein: it must play good with bodily liquids in each states. Lastly, it has to behave as a swap, altering its form relying on inputs and outputs.
Assembly all “these properties in a single protein system is difficult,” mentioned the staff.
Utilizing a mixture of AlphaFold, Rosetta, and proteinMPNN, the ultimate design appears to be like like a hinge. It has two inflexible elements that may transfer relative to one another, whereas one other piece stays folded. Usually the protein is closed. The set off is a small peptide—a brief chain of amino acids—that binds to the hinges and triggers its form change. These so-called “effector peptides” have been fastidiously designed for specificity, decreasing their probabilities of grabbing onto off-target elements.
The staff first added glow-in-the-dark set off peptides to a number of hinge designs. Subsequent evaluation discovered that the set off simply grabbed onto the hinge. The protein’s configuration modified. As a sanity test, the form was one beforehand predicted utilizing AI evaluation.
Further research utilizing crystallized constructions of the protein designs, both with or with out the effector, additional validated the outcomes. These checks additionally hunted down design ideas that made the hinge work, and parameters that tip one state to the opposite.
The take away? AI can now design proteins with two totally different states—primarily constructing organic transistors for artificial biology. For now, the system solely makes use of custom-designed effector peptides of their research, which can restrict analysis and medical potential. However based on the staff, the technique also can lengthen to pure peptides, similar to people who bind proteins concerned in regulating blood sugar, regulate water in tissues, or affect mind exercise.
“Like transistors in digital circuits, we will couple the switches to exterior outputs and inputs to create sensing units and incorporate them into bigger protein techniques,” the staff mentioned.
Examine creator Dr. Philip Leung provides: “This might revolutionize biotechnology in the identical manner transistors reworked electronics.”
Picture Credit score: Ian C Haydon/ UW Institute for Protein Design